Research Projects
-
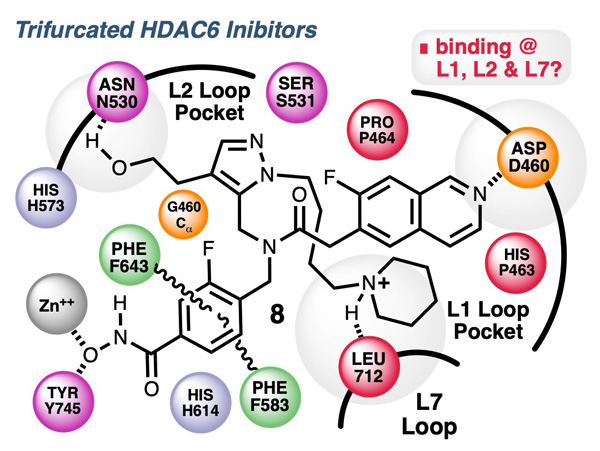 Selective HDAC6 Inhibitors to Improve Cancer ImmunotherapyThe median survival for metastatic melanoma is approximately 8–16 months, and few therapies offer a significant improvement in overall survival. However, immunotherapeutic strategies that abrogate immunologic checkpoints or improve immunosurveillance have shown promise, especially in melanoma. Genetic abrogation and pharmacologic inhibition of HDAC6 in cancer cells leads to a decreased infiltration of tumor-associated macrophages and upregulation in the expression of tumor-associated antigens and MHC class I molecules, suggesting a potential improvement in the immunogenicity of these cells. These effects translated into a pronounced delay of in vivo melanoma tumor growth, which is, at least in part, dependent on intact immunity, as evidenced by the restoration of tumor growth after CD4+ and CD8+ depletion. In collaboration with the research group of Alejandro Villagra at the George Washington University Cancer Center, our on-going research is focused on the design, synthesize, and evaluation new HDAC6 inhibitors for use in the treatment of melanoma and other malignancies.
Selective HDAC6 Inhibitors to Improve Cancer ImmunotherapyThe median survival for metastatic melanoma is approximately 8–16 months, and few therapies offer a significant improvement in overall survival. However, immunotherapeutic strategies that abrogate immunologic checkpoints or improve immunosurveillance have shown promise, especially in melanoma. Genetic abrogation and pharmacologic inhibition of HDAC6 in cancer cells leads to a decreased infiltration of tumor-associated macrophages and upregulation in the expression of tumor-associated antigens and MHC class I molecules, suggesting a potential improvement in the immunogenicity of these cells. These effects translated into a pronounced delay of in vivo melanoma tumor growth, which is, at least in part, dependent on intact immunity, as evidenced by the restoration of tumor growth after CD4+ and CD8+ depletion. In collaboration with the research group of Alejandro Villagra at the George Washington University Cancer Center, our on-going research is focused on the design, synthesize, and evaluation new HDAC6 inhibitors for use in the treatment of melanoma and other malignancies.
This research is supported by the National Cancer Institute of the NIH under award number R01CA249248-01A1 -
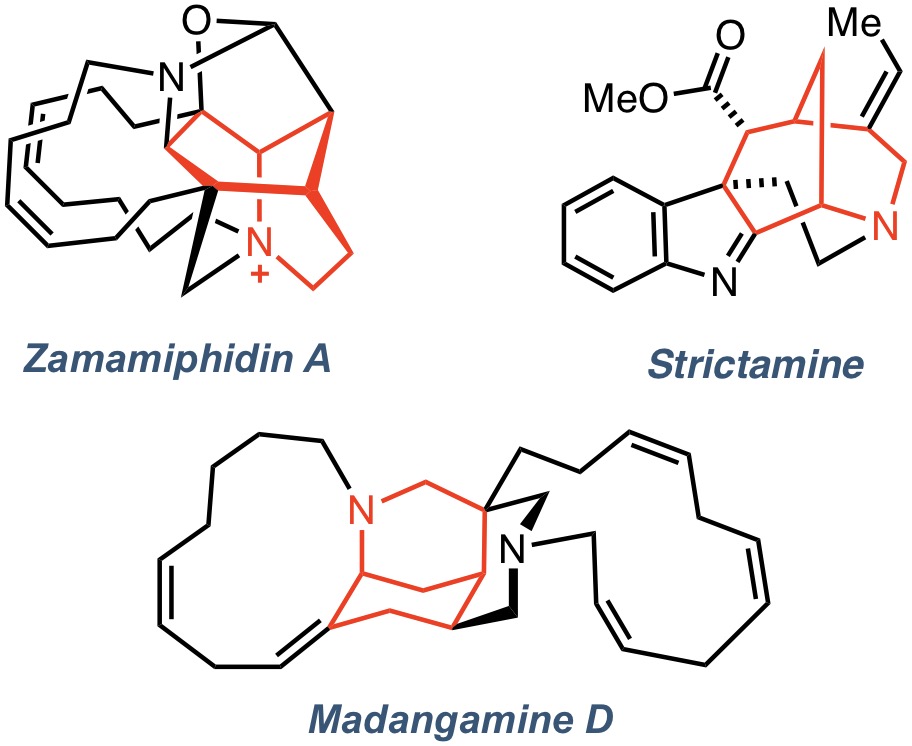 Target-Directed SynthesisIn conjunction with our interest in the chemistry of nitrenium ions and alkylidenecarbenes, we are actively involved in the total synthesis of natural products. Current targets include the alkaloids zamamiphidin A, strictamine (and other members of the akuammiline family), and madangamine D. The unifying structural feature of these complex, cage-like molecules is the 2-azabicyclo[3.3.1]nonane ring system (shown in red), which is also present in more than 300 natural products, distributed across an array of biogenetic groups that includes the Strychnos and Daphniphyllum alkaloid families. Having recently reported the development of a π+N+-type alkene oxamidation strategy for the preparation of the morphan core of madangamine D, we are now applying this approach to other targets in this group.
Target-Directed SynthesisIn conjunction with our interest in the chemistry of nitrenium ions and alkylidenecarbenes, we are actively involved in the total synthesis of natural products. Current targets include the alkaloids zamamiphidin A, strictamine (and other members of the akuammiline family), and madangamine D. The unifying structural feature of these complex, cage-like molecules is the 2-azabicyclo[3.3.1]nonane ring system (shown in red), which is also present in more than 300 natural products, distributed across an array of biogenetic groups that includes the Strychnos and Daphniphyllum alkaloid families. Having recently reported the development of a π+N+-type alkene oxamidation strategy for the preparation of the morphan core of madangamine D, we are now applying this approach to other targets in this group. -
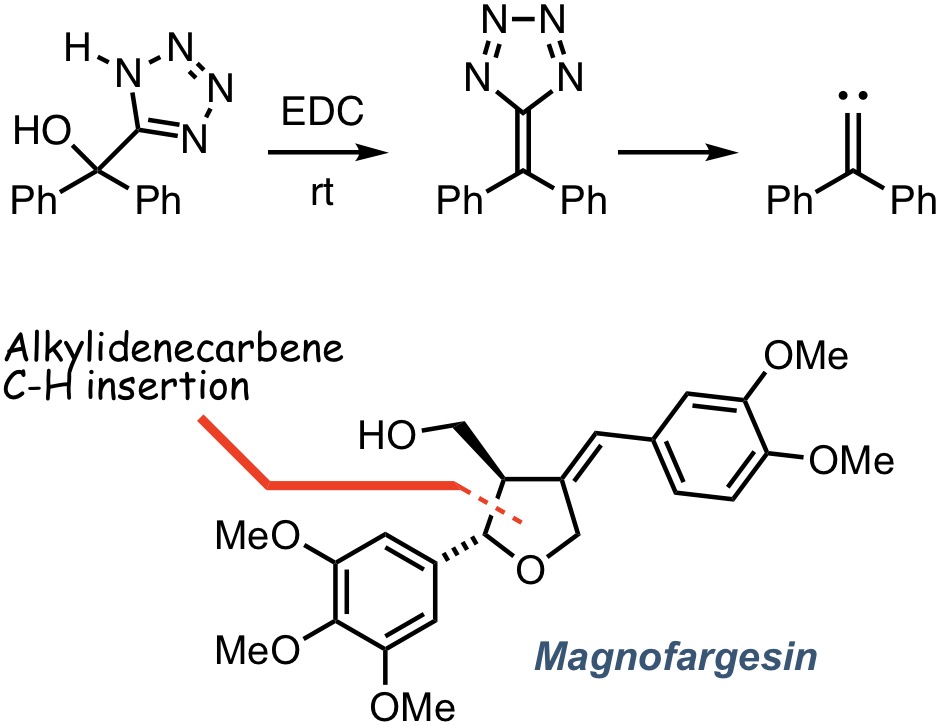 Chemistry of AlkylidenecarbenesWe have a long-standing interest in the generation and reaction of alkylidenecarbenes, transient, electron deficient species, which undergo a number of synthetically valuable reactions, including [1,2]-rearrangement, ylide formation, alkene cyclopropanation and [1,5]-C-H bond insertion. Over the last decade or so, we have studied the latter transformation as a means to access N- and O-heterocycles and natural products that encompass these ring systems, including the platelet-activating factor antagonist magnofargesin. We are also interested in the discovery of new methods for the generation of alkylidenecarbenes. In this regard, we have developed a mild, base-free method for the generation of alkylidenecarbenes involving the dehydrative fragmentation of 5-hydroxyalkyl-1H-tetrazoles. Further investigations of this unusual reaction are currently underway.
Chemistry of AlkylidenecarbenesWe have a long-standing interest in the generation and reaction of alkylidenecarbenes, transient, electron deficient species, which undergo a number of synthetically valuable reactions, including [1,2]-rearrangement, ylide formation, alkene cyclopropanation and [1,5]-C-H bond insertion. Over the last decade or so, we have studied the latter transformation as a means to access N- and O-heterocycles and natural products that encompass these ring systems, including the platelet-activating factor antagonist magnofargesin. We are also interested in the discovery of new methods for the generation of alkylidenecarbenes. In this regard, we have developed a mild, base-free method for the generation of alkylidenecarbenes involving the dehydrative fragmentation of 5-hydroxyalkyl-1H-tetrazoles. Further investigations of this unusual reaction are currently underway. -
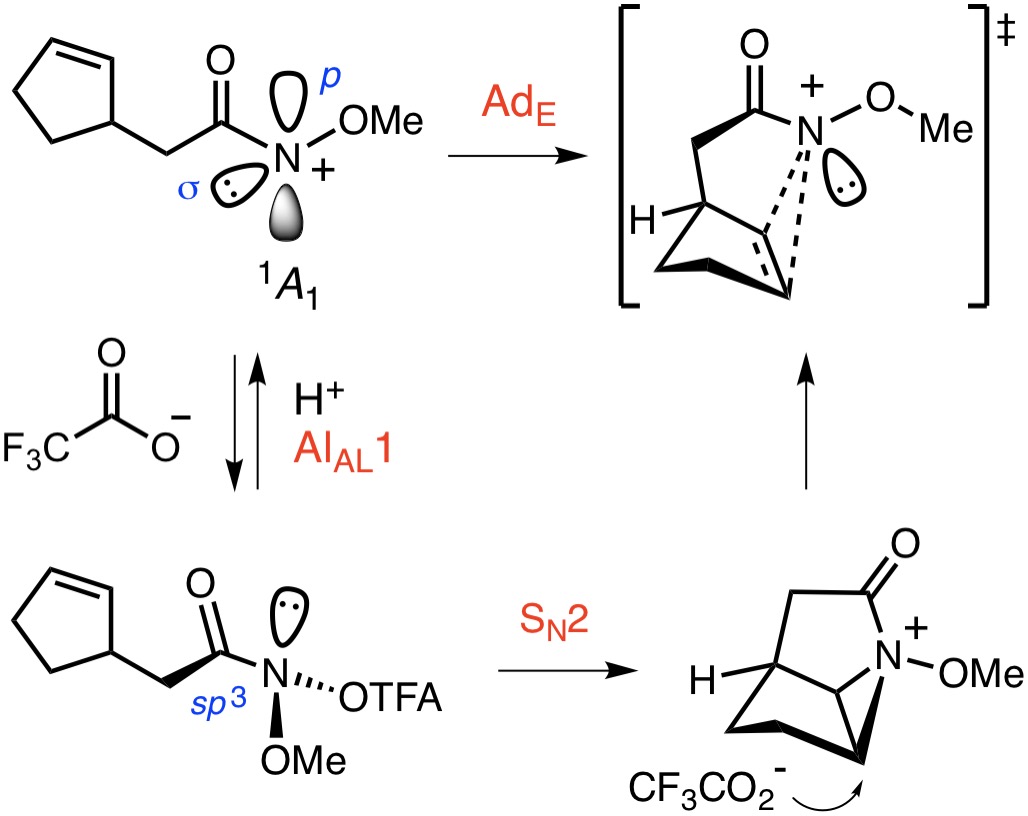 Nitrenium Ion ChemistryAlthough the existence of nitrenium ions has been demonstrated for for some time, the potential of these divalent N-electrophiles as tools for target-directed synthesis, has yet to be fully realized. Nitrenium ions have often been viewed as the poor cousins of cations, carbenes and nitrenes, their isoelectronic congeners within the electron-deficient branch of the reactive intermediate family. Our research in this area is directed towards the exploration of nitrenium ion chemistry and its application to the synthesis of saturated N-heterocycles, including alkaloid natural products. Most recently, we have reported a novel approach to the diazatricyclic core of madangamine D, which involves the π–N+-type cyclization of an unsaturated O-methyl hydroxamate. The total synthesis of madangamine D and the application of this chemistry to other morphan-based targets is underway.
Nitrenium Ion ChemistryAlthough the existence of nitrenium ions has been demonstrated for for some time, the potential of these divalent N-electrophiles as tools for target-directed synthesis, has yet to be fully realized. Nitrenium ions have often been viewed as the poor cousins of cations, carbenes and nitrenes, their isoelectronic congeners within the electron-deficient branch of the reactive intermediate family. Our research in this area is directed towards the exploration of nitrenium ion chemistry and its application to the synthesis of saturated N-heterocycles, including alkaloid natural products. Most recently, we have reported a novel approach to the diazatricyclic core of madangamine D, which involves the π–N+-type cyclization of an unsaturated O-methyl hydroxamate. The total synthesis of madangamine D and the application of this chemistry to other morphan-based targets is underway.